How DNA Sequencing Is Keeping Cancer Patients Alive Longer
As the data mounts, doctors may turn to algorithms.
Personalized therapy has been the cornerstone of cancer medicine in recent years, with many examples of big improvements in diagnosis and therapy. But more dramatic advances are on the way, as doctors translate information from multiple sources into individual treatment plans, perhaps even using computer algorithms in daily practice.
So predict the authors of “Is Personalized Medicine Here?” in the prestigious journal Oncology.
“We are entering the age of true personalized medicine,” they write.
YOU MIGHT ALSO LIKE: What Is Personalized Medicine?
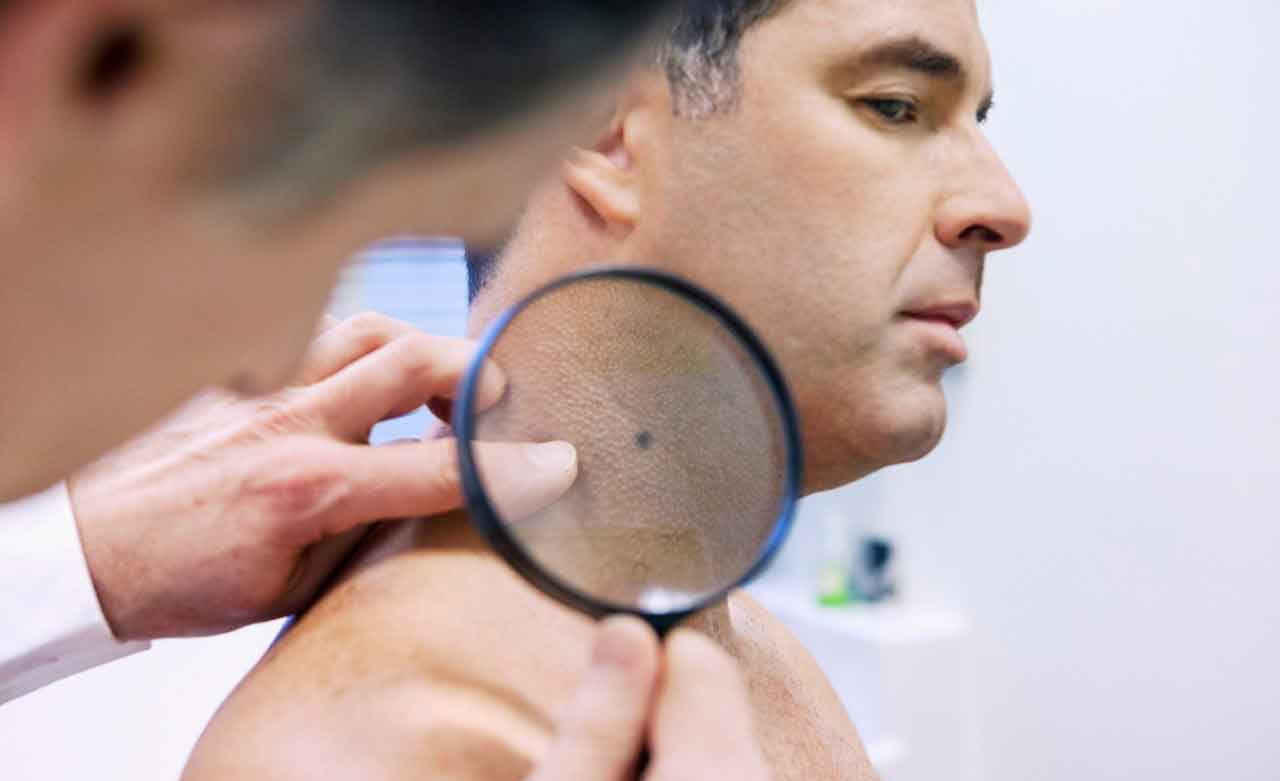
Today, a snapshot of the genes in all your cells — your overall genetic makeup — can tell your doctors something about your likelihood of developing a particular kind of cancer. We’re already well on our way with breast cancer. Women who know that certain genes run in their family — and see those genes in their own test — should go earlier and more frequently for mammograms. They are generally advised to treat their cancer more aggressively when possible cancer cells show up. We may see these kinds of tests for more cancers.
It is also often possible to sequence the genes in a particular tumor, which may give a cancer team essential information.
In the future doctors may be looking at DNA sequence profiles of your immune cells as well in order to customize your treatment. “We now have tools to define the precise defects present in each person’s tumor, and drugs that precisely target these defects…. But it turns out that each patient with advanced disease has a complex and mostly unique molecular portfolio. Therefore, in order to be ‘precise,’ we must ‘personalize” therapy, says Razelle Kurzrock, MD, from the Center for Personalized Cancer Therapy at the University of California, in San Diego.
The number of genes examined will continue to evolve. Today, doctors look at tests of 40 to 60 genes, which may be missing important information, Kurzrock notes. “With a panel of 200 genes, 90 percent of patients will have a potentially actionable alteration — a far higher number than the 20 percent often quoted for smaller panels.”
Will computers help crunch all this data and give us confidence in a treatment plan? Yes, but we can’t wait. “While there is a need for collaboration and for exploitation of computer intelligence — indeed, computers may eventually give us completely validated answers — in the meantime, it is important to realize that the practice of medicine has never depended on perfect validation, but rather on highly trained physicians making informed decisions,” Kurzrock adds.
The new treatments targeted to DNA sequencing gene results seem to be keeping patients alive longer, though not saving them. One analysis pooling many studies concluded that personalized therapy based on DNA sequencing extended median survival — meaning half died sooner and half lived longer — to more than 19 months, compared to 13.5 months for patients who received other kinds of care. However, the number of people who died from the cancer was the same.
A great deal depends on what kind of cancer you have, the stage at which it is caught, and whether tell-tale variants show up on your tests.
YOU MIGHT ALSO LIKE: Personalized Medicine for Breast Cancer
Skin cancer
These DNA sequencing tests have been especially useful for patients with late-stage melanoma, a deadly form of skin cancer, which is among the cancer that spreads fast: Stage IV patients have been living from 8 months to a year and half after diagnosis. About half of patients with a metastatic skin cancer have a mutation called BRAF V600E, which studies suggest may make their cancer spread faster. A number of recent trials have tested the effect of drugs that act directly on that mutation and related variants.
A drug called vemurafenib, for example, did so well in a multi-center trial reported in 2011 that members of the control group, who were receiving an approved chemotherapy drug instead, were switched over to join the patients receiving vemurafenib. These patients all had the tell-tale mutation and an untreated cancer. An earlier trial with patients who had already undergone treatment showed that more than half responded to vemurafenib; the median length of time researchers documented a positive response was around 7 months.
Lung cancer
Gene mutations have also led to new therapies for non-small-cell lung cancer, which accounts for about 85 percent of all lung cancers. About 10 to 40 percent of these tumors can be linked to an aberration in the epidermal growth factor receptor. Some four drugs can treat that group of tumors, with response rates as high as 80 percent. In a test of a drug called erlotinib, half of the patients who received the new drug followed by chemotherapy lived for more than 30 months. Among patients who received only chemo, the median survival rate was 11 months.
Other drugs target the 7 percent of these lung cancer patients who have a different gene mutation, keeping many of them alive a year later.
To keep the ball moving forward on personalized cancer treatment will require organizational change, says J. Leonard Lichtenfeld, MD, deputy chief medical officer for the national office of the American Cancer Society. Researchers around the world will need to collaborate more closely. “Data libraries of genomic data have to be designed, funded and made readily available. How we structure clinical trials will have to change. After all, finding those patients with a specific cancer and a specific genomic abnormality in that particular cancer is going to be increasingly difficult. No more herding in 50 patients with lung cancer at a major academic research center and try out a new drug. Now it will be a search for that patient with a specific genetic change in their cancer and hoping that — in addition to finding them — they and their doctors will be willing to participate in a clinical trial.”
Updated:
March 31, 2020
Reviewed By:
Christopher Nystuen, MD, MBA